macromolecules
A building block of life is macromolecules. Each cell in our bodies and animals' bodies contains thousands of these. Some macromolecules that use Carbon are proteins, carbohydrates, lipids, DNA, and RNA. Every macromolecule has its own "job". The job of a macromolecule depends on the Carbon backbone and the groups it has.
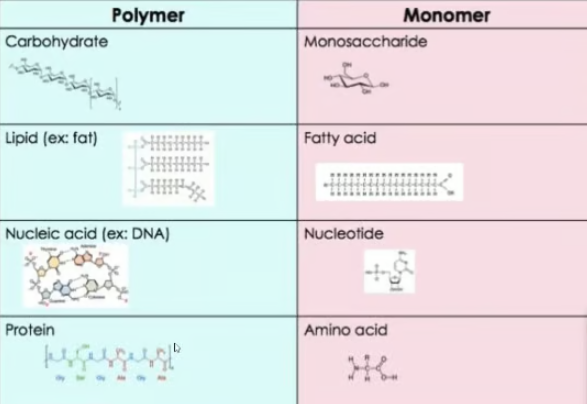
monomers and polymers
Macromolecules are split into groups of monomers and polymers. Meaning "many
monomers", polymers are made up of multiple monomers, and most macromoleceules are polymers.
Polymers can be broken up by hydrolysis (using the polarity of water to
break bonds) or combined by dehydration synthesis (removing the water to combine
things). Every macromolecule is a polymer
carbohydrates
Carbohydrates are fuel and building material and are chains of monomers. The way they
are bound changes their function and specialty, and the formation of a carbohydrate is
interconnected rings.
Glycosidic Linkage: covalent bond that connects a carbohydrate to another group of
carbohydrates.
monosaccharides
- Fuel; can be converted to other molecules or combined into polymers
- glucose
- fructose
disaccharides
These are polymers
- Formed through the synthesis of 2 monosaccharides by a glycosidic bond
- Same function as monosaccharides
- lactose
- sucrose (table sugar)
polysaccharide
- Polymer
- Long chain of monosaccharides
- Strengthens cell walls, stores glucose for energy
- cellulose (plants)
- Major structural molecule in plants (linear beta glycosidic bonds are very rigid)
- starch (plants)
- chitin (insects and fungi)
- glycogen (animals)
lipids
Lipids are a diverse group of hydrophobic molecules The formation of all lipids is chains, and they are important for energy storage and cell membranes. The function of a lipid depends on its structure
Some lipids are fats, phospholipids, and cholesterol.
- Glycerol Backbone: Contains Hydrogen, Carbon, and Oxygen. The start of all lipids
- Fatty Acids: The body of lipids and contains the same elements as the glycerol backbone but arranged differently

Fats
Fats are made up of 3 fatty acid monomers joined to a glycerol backbone by ester bonds. You may have seen these on food labels.
- Saturated Fats
- The acids are packed tightly together, making them solid at room temperature
- Store the most energy
- Unsaturated Fats
- Due to the presence of a double bond, the acids are not packed tightly together, making them liquid at room temperature
- Easier for your body to break down
Phospholipids
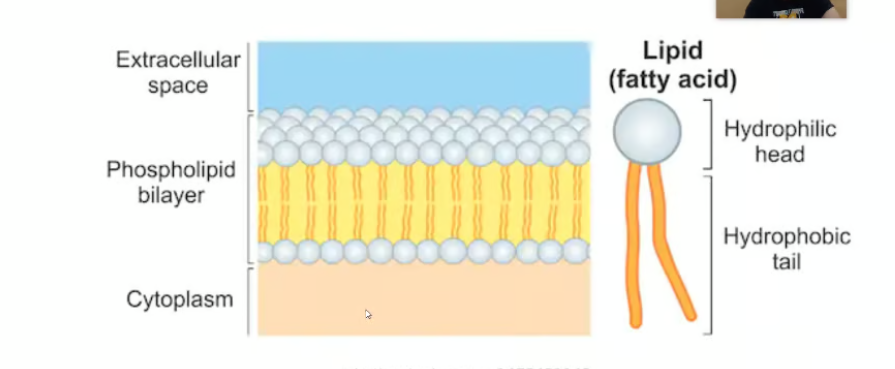
These make up strong cell membranes and each one has a hydrophilic (water attracted) head and hydrophobic (water repelling) tail. When phospholipids are added to water, they self-assemble into a bilayer, with the hydrophobic tails pointing toward the interior.
Cholesterol
- Steroid
- Interconnected C-ring formation
- Found in cell membranes, myelin sheath
- Maintains membrane fluidity and structural support
- Precursor to vitamin D and steroid hormones like testosterone, estrogen, cortisol
proteins
Have many functions
- Regulate reactions as enzymes
- Form muscle and other structures
- Transport substances into and out of the cell
amino acids
- Monomers
- Bonded by peptide bonds
- The R-group an amino acid has determines its structure and function. There are 20 different types of R-groups
- Polypeptide chains: chains of amino acids and a precursor to proteins. A polypeptide chain is the linear sequence, while the protein is the functional and folded 3-D structure
levels of protein organization/structure.

- Primary structure: Linear sequence of amino acids
- Secondary structure: Regular/repeated configurations caused by H-bonding
- Tertiary structure: Intricate 3-D structure from interactions between R-groups; greatly impacted by earlier levels
- Quaternary structure: Structure formed from interaction of 2 or more polypeptides
denaturation
- Alterations in pH, salt concentration, temperature, or other environmental factors can cause a protein to unravel. As a result the protein loses its structure and function 😢
nucleic acids
Nucleic acids (the polymers) are made up of a chain of nucleotides (the
monomers)
- DNA
- Deoxyribonucleic acid
- This is the genetic material of all living organisms. Primary part of our chromosomes
- RNA
- Ribonucleic acid
- This transcribes (copies) the genetic info in DNA and turns it into proteins
A phosphate group is a phosphorus atom bonded to 4 oxygen atoms. It's crucial as it's the backbone of RNA and DNA.